The Arena da Amazônia in Manaus, Brazil, was a fatal arena. All teams featuring there in the 2014 FIFA World Cup fell under the unbearably heat. Italy, England, Cameroon, Croatia, USA, Portugal, Honduras, Switzerland. All lost their game following that at Manaus (with the exception of Portugal who won over a Ghana team on strike and Honduras who got eliminated after their Manaus game…).
It’s hard to say. But I doubt it. Italy, England and Croatia were all favorite for their next games, but they all seemed short of energy in the last minutes.
Weirdly enough… no. As teams played late in the evening, the temperature never exceed 32°C (89.6°F). As a result, accordingly to the FIFA cooling break rule, players were not given any additional break to recuperate from the exhausting heat. Nevertheless, this didn’t prevent players from making a big deal out of Manaus’ unbearable climate.
Italian footballer and goalscorer Claudio Marchisio even asserted: “At times it seemed we were having hallucinations from how hot it was”…
Good question! In short, it’s because what you feel is the dynamics of heat, also known as thermodynamics. But not heat itself.
You Do Not Feel Heat Nor Cold
Let me say it once again, slightly more rigorously. You cannot feel temperature.
If you’ve been to the beach last summer, you should have an idea of what I’m talking about.
Did you swim? Have you experienced that refreshing (if not scary) jump from the sand to the water? Has it ever occurred to you just how cold water was?
In summer it is indeed. But weirdly enough, that water is still usually above 20°C (70°F).
In the spring or the fall, you’re probably hanging around in tee-shirts at that temperature. Yet, you may not feel cold. In fact, if you’re running or doing sports at 10°C, you’ll probably be fine even if you’re topless.
My point is that 20°C (70°F) water feels much colder than 20°C (70°F) air.
Well, let’s think about it. Have you ever been into a jacuzzi and in a sauna? While they might both feel as hot as one another, the jacuzzi never exceeds 40°C (104°F) for health reasons, while saunas go up to 80°C (175°F)! Somehow, at high temperature, it seems to be the exact opposite. Water seems hotter than air! Similarly, here’s an advice: never use metallic objects to take out a plate from the hot oven. They’ll feel much hotter than, say, wood.
As I stressed it earlier, what you feel is not heat. But instead of explaining why myself, check this awesome video by Derek Müller on Veritasium. I think it’s one of the very best of Derek’s.
So there it is. 20°C (70°F) metal feels colder than 20°C (70°F) paper, because it absorbs heat away from you (or whatever is in contact with it) at a faster rate than paper does. This rate of heat absorption (or loss) is precisely what you feel, as opposed to the actual temperature of objects you interact with.
It kind of does! Water, like metal, conducts heat quicker than air, or paper, does. Now, another important aspect to keep in mind is that your body temperature is at 37°C (98.6°F). So, if we enter in contact with anything of smaller temperature, heat will be driven away from us. Conversely though, when we touch something of greater temperature, like the water of jacuzzis or the air of saunas, then heat will run into our body. Now, because water conducts heat quicker, jacuzzi water drives heat into your body at the same rate as the hotter sauna air does. This is why 40°C (104°F) jacuzzis feel as hot as 80°C (175°F) saunas.
Exactly! Now, a clever way to think about temperature is to notice how misleading the adjectives “hot” and “cold” are. In the following video on Minute Physics, Henry Reich proposes to redefine “hot” as “the property of objects to give away heat”, while “temperature” is “a measure of the jiggliness of particles of an object”.
Following Henry’s definition, while there’s a correlation between “heat” and “high temperature”, they are far from being the same…
Something will feel hot if it infuses heat into your body. The more it does that, the hotter it feels. Conversely, something will feel cold if it absorbs heat away from your body. And the more it does that, the colder it feels.
For a given material, the higher its temperature the hotter it will feel. But, crucially, for different materials, the more the material conducts heat, the more extreme it will feel (either hotter if its temperature is greater than body temperature or colder otherwise).
Hummm… I should also say… Should I say it?
Fine, I’ll say it. I’ve kind of just lied to you. There are particular settings of extreme physics where very low temperature objects are actually… hot!
I shouldn’t go into details right here, but basically, some low temperature objects tend to give away energy to any object in their surrounding! This is explained by Professor Moriarty on Sixty Symbols:
Wind and Humidity
Now, as you’ve probably guessed, wind and humidity don’t actually affect the temperature of the air surrounding us.
Exactly! Let’s start with humidity as it is simpler. Basically, to have an idea of the effect of humidity on conductivity, a clever trick that physicists like to abuse of is to consider the extreme case. And the case of extreme humidity is quite simple: It happens when you substitute air by water entirely! This gets us back to the sea vs jacuzzi situation. As we have seen, 20°C (70°F) sea water is much colder than 20°C (70°F) atmospheric air, while 40°C (104°F) jacuzzi water is much hotter than 40°C (104°F) air. This is because water has a greater thermal conductivity than air.
Yes. So, the more water in the air, the more thermally conductive the humid air! So, if the air temperature is hotter than 37°C (98.5°F), then humidity will make air feel even hotter. Meanwhile, if it is (very) cold, then humidity will make air feel even colder.
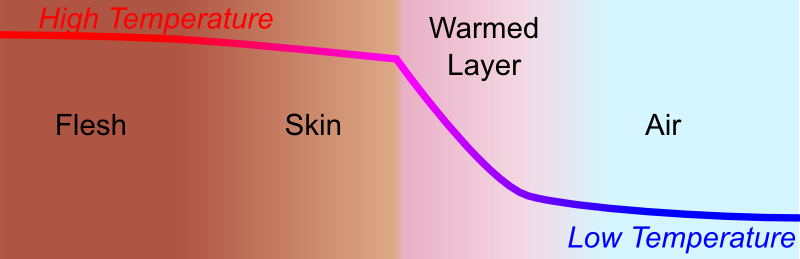
It’s a bit trickier. But a simplified way to understand its effect is to look more closely and the thermal interactions between your skin and the air, when the air is not moving. Let’s draw a sketch in the case where air is colder than 37°C (98.6°F).
Now, here’s the important bit. When air is not moving, a layer of warmed air forms around the skin. As a result, temperature decreases relatively smoothly from the flesh to the air. But then, at the immediate contact between skin and air, the difference of temperature is quite small, and hence the flow of heat from the skin outwards will be small. Thus, we will not feel too cold.
Exactly. And, as a result, when wind blows, the air in contact with our skin is constantly renewed, hence preventing the formation of a thick layer of warmed air that would prevent heat from flowing away from our body at too fast a rate.
In fact, more generally, the key to not being cold when the air temperature is very low, is to construct a thick layer of warmed air around our body.
As often, the answer can be found in Nature. In fact, Nature had figured that out millions of years ago, when she invented hair and feather.
Have you ever had goosebumps on a cold winter evening?
Well, that’s Nature straightening your hair to trap a thick layer of hair on the surface of your skin! This is beautifully explained by AsapSCIENCE in the following video:
Now, we clever human beings have gone further and created clothes. The role of clothes is exactly the same as goosebumps, although clothes are evidently much more effective. They trap air around your skin, hence forming a thicker warmed layer. At last, this diminishes the flow of heat out of our skins. In other words, it makes us feel less cold!
Yes! Civil engineers have found out that window glasses had a high thermal conductivity. As a result, heat tends to flow out of buildings through windows, even when windows are closed. They then noticed that, conversely, air has a low thermal conductivity. Their clever idea was to form a thick layer of warmed air, which they put in-between two window glasses. This technique is called double glazing, and if your house is modern, it probably has it!
Phase Transitions
Still, if we look back to the initial example of this article, it seems that our journey through the physics of temperature needs an important halt.
Manaus, that city that terrified so many FIFA World Cup football players, is very humid, but its temperature hardly exceeds 32°C (89.6°F). So…
You’re right! Humidity does cool players.
It’s because thermal conductivity is not the only thing that affects heat transfer!
Take some drink. If it’s not cold enough, add some ice. Now, here’s a tricky question: Why will your drink cool down?
Well, not really. In fact, if you measure the temperature of the ice, you’ll see that it doesn’t warm up. It remains at 0°C (32°F), even while the temperature of water is decreasing. Similarly, I don’t know if you’ve ever done the experiment, but the temperature of boiling water in your casseroles will never exceed 100°C (212°F).
Exactly! The transition from solid to liquid and the one from liquid to gas are ways for materials to absorb energy. Quite a lot of it, actually. So, when ice melts in your drink, it absorbs the thermal energy of your drink to become liquid. This absorption is about 10 times more important than the effect of thermal conductivity. It is this huge factor that explains why muggy 30°C (86°F) Manaus is so terrifying!
When players run, their internal temperature increases. It is then of the greatest importance that they succeed in getting this temperature increase out of their body. As you know, they do so by sweating.
It’s not the sweating per se that cools them down, but the evaporation of the sweat. By undergoing a phase transition, sweat takes energy away from our bodies, which cool them down.
The trouble with humid air is that it’s already filled with water vapour. And this implies that the air is not really willing to accept more water vapour. And, thus, while players sweat a lot, their sweats hardly evaporates. And that’s why their bodies have trouble to cool down.
Exactly! Because the body is hot it keeps sweating. However, because the air is already too humid, the sweat does not evaporate. This means that we end up too hot, all sweaty, and very sticky because of the sweat…
Oh yes! Also, hut muggy weather also often leads to thunderstorms… Hot muggy weather… I hate it so much!
Let’s Conclude
What I love about the physics of temperature is how counter-intuitive it is. Evidently how we feel about temperature has to do with the temperature of our surrounding. But importantly, this temperature of our surrounding is not what we feel. What we ultimately feel is rather the outflow of temperature from our body. This outflow can be a thermal transfer that will affect the temperature of our surrounding, or modify the state of the material we are in contact in, by, for instance, making sweat evaporate. It is important to understand these things to better anticipate the effect of weather conditions on humans’ health. And to, for instance, give well-deserved cooling breaks to the sweating football players in Manaus…





Thank you so much for this article, I really learned a lot from it.
I do have a question which relates to different body types. Some people feel cold at higher air temperatures than others while some people are more affected by humidity than others. Can you explain this please?
Nice article (as usual), but I do have a quibble. While it is true that the *temperature* of another object or of our surroundings is not what we feel, I suspect that what we do feel is either or both of the heat within us and the heat flow through our skin. And since some physicists make a point of identifying heat only with flow rather than total internal disorganized kinetic energy, it might be argued that heat in that sense is exactly what we *do* feel.